Vero Cell Vaccine Duration Between Two Doses / Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu
China donated Covid-19 vaccine Vero Cell arrives in Kathmandu. Efficiency Against the New Mutations of.

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld
So the first dose it starts your immune system making those.
Vero cell vaccine duration between two doses. The most common local adverse reaction in both groups was pain at the injection site 4. 2 doses 05 mL each at a recommended interval of 3 to 4 weeks. In light of the observation that two-dose efficacy and immunogenicity increase with a longer inter-dose interval WHO recommends an interval of 8 to 12 weeks between the doses.
A review paper in the British Medical Journal based on 17177 vaccinated people in the United Kingdom Brazil and South Africa support the British. Vero cell vaccine duration between two doses. Cell-based antigen production may offer a faster and more stable production of vaccines compared to embryonic chicken eggs which produce 1-2 vaccine doses per chicken egg.
Indian govt revises gap-duration between two doses of Covid vaccine. 600 for private hospitals. Vaccine doses as low as 375 µg or 75 µg are highly immunogenic following two dose immunization as shown in the MN and the SRH test Non-adjuvanted formulations are more immunogenic than the adjuvanted ones Vaccine induced substantial immune responses in immunocompromised and chronically ill individuals.
Two doses with four to 8 weeks in between. 600 for states and at Rs. The local and systemic adverse reactions were compared between two groups.
Vaccine Placebo NMPA definition 854953110 1684870223 507 359 620 Protocol definition 804953104 1704870227 541 401 648 As of 16 th Dec 2020 the average follow-up time after the 2 nd dose was 703 256 days and the median follow-up duration was 730 days. 1200 for private hospitals. The vaccine is available at Rs.
Though host cells replicate well in chicken eggs vaccine production with mammalian cells would not rely on an adequate supply of chicken eggs to produce each vaccine. The ministrys clarification came in. 6th month and 12th month after 2 doses of immunization.
While studies are ongoing regarding the interchangeability between vaccines more evidence is needed on the interchangeability of a second dose of AstraZeneca with Vero Cell vaccine before conclusions can be made reads the statement. The incidence of severe cases of SARS-CoV-2 pneumonia and deaths accompanied by COVID-19 after two-doses of vaccination Time Frame. Most of the reported systemic.
From 14 days after the second dose to 14 days after the third dose the levels of neutralizing antibodies of these two groups continued to rise and this trend remained until 14 days after the fourth administration showing that the SARS-CoV-2 inactivated vaccine Vero cells could stimulate Sprague Dawley rats to produce a humoral immune response and the antibody is maintained at a. Minimum interval between a dose of COVID-19 vaccine and any other dose of vaccine is from 2 4 weeks depends on the vaccine type. The World Health Organization WHO recommends an interval of.
21 to 28 days after first dose. 400 for states and at Rs. It is also reassuring to find that the majority of T cell responses in recipients of two doses of the BNT162b2 vaccine are generated by epitopes that are invariant between the prototype and two of.
The UN health agency said that data on the efficacy of the mix and match of vaccines remains limited. In addition cell-based vaccines may allow for multiple viral vaccines. The second does is just a booster shot its exactly the same thing as what was in the first dose same vial theres no difference.
The mean age was 268 years SD 131 years and 274 years SD 139 years in PVRV and PCECV group respectively. The vaccine is available at Rs. From14 day after the second dose to 6 month after the second dose The incidence of any adverse reactionsevents Time Frame.
If the second dose is inadvertently delayed beyond 4 weeks it should be given. Currently both the Pfizer and Moderna COVID-19 vaccines require two shots to be fully effective. Number and percentage of local and systemic adverse events occurring within 7 days after a booster dose of inactivated Vero cell culture--derived Japanese encephalitis vaccine JE-VC manufactured as Ixiaro administered 15 months after dose 1 of a 2-dose primary JE-VC series.
At the start date Dose 2. In this clinical trial low dosage 300SUdose and medium dosage 600SUdose with two-dose immunization scheduled at 28-day intervals will be adapted. GMT of anti-SARS-CoV-2 neutralizing antibody at 6 and 12 months Time Frame.
Secondary efficacy endpoints are the incidence of hospitalizationmortality rates among one or two dose regimens duration of immunogenicity rates up to 120 days the seroconversion rate the seropositivity rate neutralizing antibody titer and IgG levels 14 days after each dose of vaccination. Two doses with four to 6 weeks in between. Is there a difference between the first and second COVID-19 vaccine doses.
If the second dose is inadvertently administered earlier than 3 weeks after the first the dose does not need to be repeated. On February 10 an expert panel of the World Health Organization suggested an 8-12 week interval between the two doses. What is the time interval between taking COVID-19 vaccine and any other vaccine.
If the second dose is inadvertently administered less than four weeks after the first the dose. Bites were most often located on the lower extremities in both groups.

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
Sinopharm Vaccine Side Effects Price Efficacy Of China S Sinopharm Covid Vaccine India News Times Of India

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

Integrated Control Of Covid 19 In Resource Poor Countries International Journal Of Infectious Diseases
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
Singapore Excludes Sinovac Shots From Covid 19 Vaccination Tally Nikkei Asia

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
Vaccines Free Full Text Development And Evaluation Of Vero Cell Derived Master Donor Viruses For Influenza Pandemic Preparedness Html

Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use
Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 4 Sage29apr2021 Sinovac Pdf
Https Www Who Int Docs Default Source Coronaviruse Act Accelerator Covax 21221 Sinovac Vaccine Explainer Pdf Sfvrsn 69283a08 3 Download True
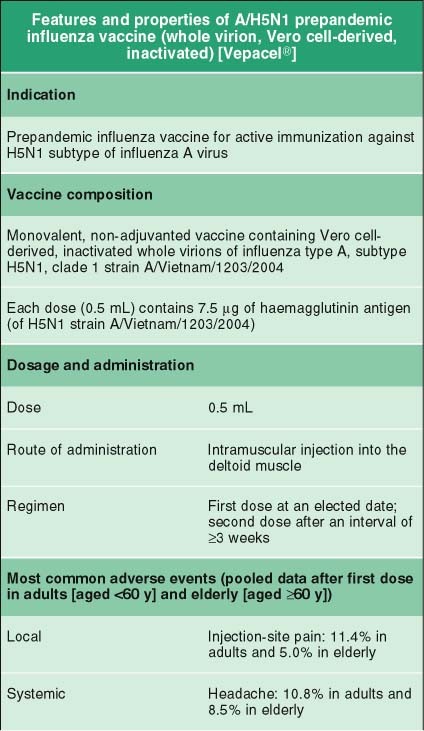
A H5n1 Prepandemic Influenza Vaccine Whole Virion Vero Cell Derived Inactivated Vepacel Springerlink

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases
Sinovac Covid 19 Vaccine Safe For Ages 3 To 17 In Small Early Trial Devex