Vero Cell Vaccine Effectiveness | Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld
95 cases in placebo group. Suspension for injection Presentation.

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases
Not applicable Route of administration.
Vero cell vaccine effectiveness. Purified Vero cell rabies vaccine is safe carries a very low adverse reaction rate and is effective in preventing rabies in severely exposed subjects when used with human or equine rabies. Cells used for vaccine manufacture are the working Vero cell bank which is of the 142nd generation. Latin American and Asian countries have also allowed the use of Vero Cell.
The WHO says the Chinese vaccine is safe effective and of good quality. The use of cell-culture technologies for the manufacture of influenza vaccines might contribute to improved strain selection and robust vaccine supplies. The results showed that the participants vaccinated with medium dose 600SU and high dose 1200SU vaccines for 28 days and then the positive seroconversion rate reached to over 941.
Not applicable Vaccine Vial Monitor. Preliminary results of laboratory studies and clinical studies will show that the vaccine produces antibodies that. The Chinese vaccine is prescribed to those over 18 years of age in two doses.
Protective effect against COVID 19 after 14 days following the full course of vaccination among healthy population aged 18 years old and above. The above studies showed that the medium dose high dose and 014 and 028 procedures can produce a certain level of protective antibodies. Vero cell vaccine covid-19 effectiveness.
Veros unique pipeline streamlines the inactivation harvest and purification of large batches of viruses within robust safety margins at reasonable costs. Special conditions for storage. Vialnon-auto disabled single use prefilled syringe Number of doses.
We investigated the safety immunogenicity and protective efficacy of a Vero-cell-culture-derived influenza vaccine and assessed the correlation between vaccine efficacy and haemagglutination inhibition antibody titre. The platform has already been utilized to rapidly generate novel vaccines for several viral epidemics. 10 11 Peer-reviewed results published in JAMA of Phase III trials in United Arab Emirates and Bahrain showed BBIBP-CorV 781 effective against symptomatic cases and 100 against severe cases 21 cases in vaccinated group vs.
01 June 2021 Product Description Pharmaceutical form. COVID-19 Vaccine Vero Cell Inactivated also contains an adjuvant a substance that helps strengthen the immune response to the vaccine. The Vero Cell vaccine was developed by the pharmaceutical company Sinovac Life Sciences whose applicant in the European Union was LifeOn.
Vero cells vaccine efficacy. The primary objective was the efficacy of the vaccine in preventing cell-culture-confirmed influenza infection with viruses that were antigenically matched to one of the vaccine strains. Public health authorities across the globe have been mobilising to deliver the biggest ever vaccination programme to battle Covid-19.
The study found no deaths related to COVID-19 in patients who received both doses. The vaccine was. Pandemic influenza vaccine in humans.
The Vero cell platform vaccine production technology addresses precisely this need. Safety and efficacy of purified Vero cell rabies vaccine given intramuscularly and intradermally. Vero cell vaccine effective.
Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru. What is the effectiveness of two doses of inactivated Vero cell-derived JE vaccine in preventing JE disease in individuals living in JE-endemic areas. So far Vero is the only Chinese vaccine for which the manufacturer has published official data.
Vero cell vaccine efficacy china. A Vero Cell-Derived WholeVirus H5N1 Vaccine Effectively Induces Neuraminidase- -Inhibiting AntibodiesJ. On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation.
The governments decision to administer the Vero Cell vaccine developed by Chinas Sinopharm is not the right choice of vaccine for migrant workers stakeholders say. When a person is given the vaccine their immune system identifies the inactivated virus as foreign and makes antibodies against it. China joined this club of elites of science following the Chinese FDA approval of Sinopharm the subsidiary of state-owned China National Pharmaceutical Group- CNPG first COVID-19 vaccine inactivated Sars-Cov-2 based on the results of the phase-3 clinical trial in UAE and Bahrain showing up to 86 efficacy of the vaccine in preventing COVID-19.
A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Purified Vero cell rabies vaccine is safe carries a very low adverse reaction rate and is effective in preventing rabies in severely exposed subjects when used with human or equine rabies immune globulin. Rating Adjustment to rating.
Vero cell covid vaccine effectiveness.

Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
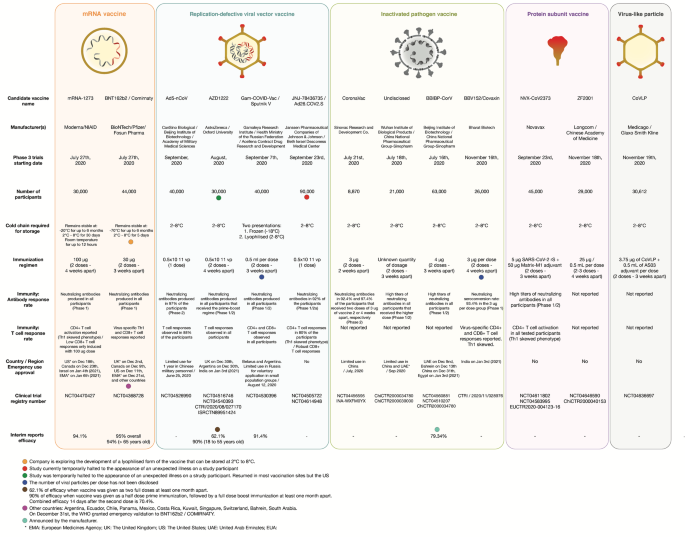
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Vaccines Free Full Text Development And Evaluation Of Vero Cell Derived Master Donor Viruses For Influenza Pandemic Preparedness Html
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December
Https Www Who Int Immunization Policy Position Papers Je Grad Inactivated Effectiveness Pdf
World Health Organization Who What S The Difference Between Covid 19 Vaccine Efficacy And Effectiveness Vaccine Efficacy Refers To How The Vaccine Performs In Ideal Conditions Controlled Clinical Trials Vaccine

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

Seychelles Brings Back Curbs Despite Vaccination Success Bbc News

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters
Expert Chinese Vaccines Effective Against Covid 19 Delta Variant Cgtn
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu

Efficacy Safety And Immunogenicity Of A Vero Cell Culture Derived Trivalent Influenza Vaccine A Multicentre Double Blind Randomised Placebo Controlled Trial The Lancet

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld
